Alongside W. Edwards Deming’s development of the Plan Do Study Act (PDSA) cycle, the Juran Trilogy – Quality Planning, Quality Control and Quality Improvement – forms the basic building blocks for Quality Management Systems. This article draws from Juran’s Quality Handbook, Fifth Edition, which I would encourage NHS colleagues to read. The page numbers in brackets refer to its relevant pages.
Of the many meanings of the word quality, two are of critical importance to managing for quality:
1. Quality means those features of products or services which meet customer needs and thereby provide customer satisfaction. In this sense, the meaning of quality is orientated to income. The purpose of such higher quality is to provide greater customer satisfaction and, one hopes, to increase income. However, providing more and/or better quality features usually requires an investment and hence usually involves increases in cost. Higher quality in this sense costs more.
2. Quality means freedom from defects – freedom from errors that require doing work over again (rework) or that results in field failures, customer dissatisfaction, customer claims, and so on. In this sense, the meaning of quality is orientated to cost, and higher quality usually costs less (page 2.1).
Figure 2.1 elaborates on these two definitions.
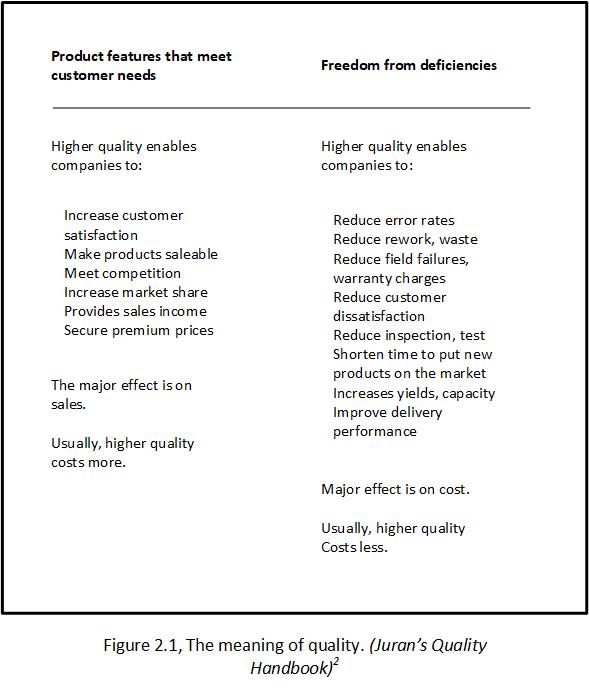
Income may be assumed to be less relevant to the NHS than to commercial businesses but this is not the case. Higher income could be achieved by attracting more patients to the high quality services that you offer and more Invitations to Tender (ITT) from your Clinical Commissioning Group (CCG).
While patients are indeed customers in this context, there are other customers to be considered such as your CCG. ‘Freedom from defects and error’ is better understood in the NHS as patient safety and the Health Foundation’s publication, The measurement and monitoring of safety (see Resources section below), provides a helpful framework. ‘Cost’ is the additional expense to the Trust as a result of rework and litigation for example.
Quality Management – prime focus
- Achieving results that satisfy the requirements for quality.
- Motivated by stakeholders internal to the organisation, especially the organisation’s management.
- Goal is to satisfy all stakeholders.
- Effective, efficient, and continually improving, overall quality related performance is the intended result.
- Scope covers all activities that affect the total quality-related business results of the organisation.
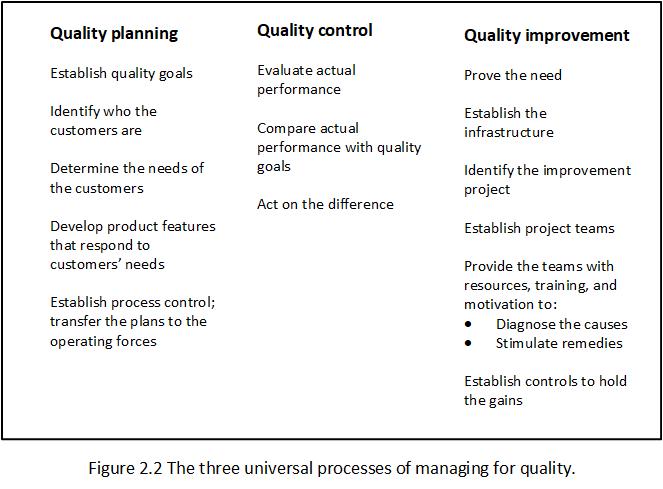
The universal processes of managing quality are Quality Planning, Quality Control and Quality Improvement. These three processes are referred to as the Juran Trilogy. See Figure 2.2 (page 2.6).
Definitions
Quality Planning – is a structured process for developing products (both goods and services) that ensures that customer needs are met by the final result.
Quality Planning steps:
- Establish the product or service.
- Identify the customers.
- Discover the customer needs.
- Develop the product or service.
- Develop the realisation process.
- Develop the controls and transfer to operations.
Quality Control – is a universal management process for conducting operations so as to provide stability – to prevent adverse change and to ‘maintain the status quo’.
To maintain stability, the quality control process evaluates actual performance, compares actual performance to goals, and takes action on the difference. Quality Control is achieved via a Process Management System (PMS).
Quality Improvement – as used here, ‘improvement’ means ‘the organised creation of beneficial change; the attainment of unprecedented levels of performance.’ A synonym is ‘breakthrough.’
There are two kinds of beneficial change. Better quality is a form of beneficial change. It is applicable to both kinds of beneficial change that are summarised below (page 5.3):
- Quality Improvement to increase customer satisfaction may consist of such actions as:
- product or service development to create new features that provide greater customer satisfaction and hence increase income
- business process improvement to reduce the cycle time for providing better service to customers
- creation of ‘one-stop shopping’ to reduce customer frustration over having to deal with multiple personnel to get service.
2. Quality improvement to reduce deficiencies that create chronic waste may consist of such action as:
- increase of the yield of factory processes
- reduction of the error rates in the offices
- reduction of field failures.
The end result, in both cases, is called Quality Improvement. However, the processes used to secure these results are fundamentally different (page 5.3).
‘Improvement’ means ‘the organised creation of beneficial change; the attainment of unprecedented levels of performance.’
Quality Improvement to increase income starts by setting new goals, such as new product or service features, shorter cycle times, and one-stop shopping.
Meeting such new goals requires several kinds of planning, including Quality Planning.
Quality Planning is done through a universal series of steps:
- Identify the customers who will be affected if the goal is met.
- Determine the needs of those customers.
- Develop the product or service features required to meet those needs, and so on.
Collectively, this series of steps is the Quality Planning roadmap.
In the case of chronic waste, the product or service goals are already in place; so are the processes for meeting those goals. However, the resulting products or services do not all meet the goals. Some do and some do not.
As a consequence, the approach to reducing chronic waste is different from the Quality Planning roadmap. Instead, the approach consists of:
- Discovering the causes – why do some products or services meet the goals and others do not?
- Applying remedies to remove the causes.
This is called the universal sequence for quality improvement.
These two Quality Improvement processes require different approaches to identify the beneficial change required. The first type of Quality Improvement uses the Quality Planning roadmap, Figure 3.2 (Page 3.3). The second type of Quality Improvement uses the Universal Sequence for Quality Improvement, Figure 4 (Page 5.39).
(Notes: ‘Yield of factory processes’ relates to throughput of a Trust’s service processes. ‘Chronic waste’ relates to rework and over use of resources.)
Quality Assurance – prime focus
- Demonstration that the requirements for quality have been met (and can be) achieved.
- Motivated by stakeholders, especially customers, external to the organisation.
- Goal is to satisfy all customers.
- Confidence in the organisation’s products is the intended result.
- Scope of demonstration covers activities that directly affect quality-related process and product results.
This is achieved by evidence of the application and results of Quality Planning, Quality Control and Quality Improvement.
The anatomy of Quality Assurance is very similar to that of Quality Control. Each evaluates actual quality. Each compares actual quality with the quality goal. Each stimulates corrective action as needed. What differs is the primary purpose to be served.
Under Quality Control, the primary purpose is to serve those who are directly responsible for conducting operations – to help them regulate current operations.
Under Quality Assurance, the prime purpose is to serve those who are not directly responsible for conducting operations but who have a need to know – to be informed as to the state of affairs and, hopefully, to be assured that all is well (Page 4.3).
Keep an eye out for the third blog in this series, which will discuss in more detail how Juran’s Trilogy can be applied in a health care setting. Take part in ongoing discussions on these topics and find out about upcoming events in our Quality Management in Healthcare Special Interest Group.
Comments
Nigel Cols 31 Jan 2023
Excellent blog Tom, the key for me has always been in defining "quality" and once you've decided to keep it firmly in your mind.